February 2022 has been an important month in the battle against cancer. A recent paper published in Nature discloses the long-term results of CAR T-cell therapy treatments delivered to a leukaemia patient in 2010. More than ten years later, the immune cells continue to patrol the patient’s blood and he remains in remission.
“We can now conclude that CAR T cells can actually cure patients with leukaemia,” Dr. Carl June told reporters at a press briefing describing results that were published in Nature on February 2, 2022.
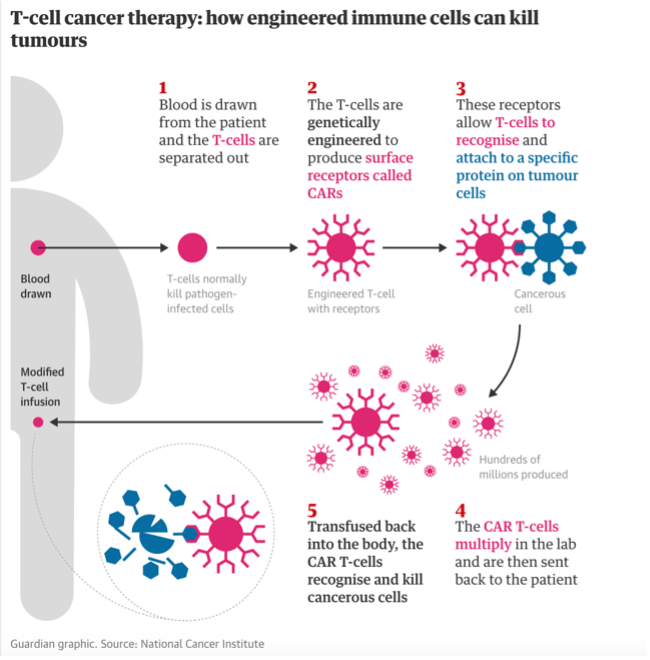
The Guardian also shared the story that created tremendous excitement for cancer researchers and patients worldwide.

Most in oncology drug development are aware of the promise of immunotherapies – those in the clinic and the few now approved by the FDA, EMA and PMDA. T-cell transfer therapy is a very promising type of immunotherapy treatment that involves teaching healthy immune cells how to identify and destroy cancer cells.
In addition to liquid tumors, researchers are exploring how to deliver this promising CAR T-cell therapy treatments to solid tumors. Medelis discusses recent applications for glioblastoma, thyroid cancer and breast cancer here.
Medelis has been managing CAR T-cell therapy studies since 2015. However, since we’re still in the early stages of studying CAR T-cell therapies in the clinic, many sites, CROs and clinical operations folks are still learning about the best approaches for keeping patients safe.
Medelis has been working with sponsors on promising phase I and phase II studies. In phase I, it’s critical to properly determine the dose. Cytokine release syndrome can cause life-threatening side effects. Other serious side effects include:
Planning and continued communication are the keys to mitigating risk – to know what to watch for and communicate with the CRAs and cancer care team.
It’s also critical for the CRO or study management team to have the proper education and procedures in place to be able to recognize and treat these before they become life-threatening.
Find more tips about mitigating issues with immunotherapy studies in the clinic:
Managing the Side Effects in a CAR T-cell Therapy Study
Common Causes of Delays in Immuno-Oncology Studies
Therapies That Might Affect the Cancer-Immunity Cycle
Guidelines for the Evaluation of Immune Therapy Activity
Monitoring irAEs and SAEs in Oncology Immunotherapy Studies
Site Selection for Cancer Immunotherapy Studies
Contact us to discuss your study. For our audience in China, follow us on WeChat here:
