Checkpoint inhibitors, including those targeting PD-1 and PD-L1, comprise one of the most promising areas of cancer therapeutics currently in development. PD-1 and PD-L1 inhibitors work by disrupting the signal that cancer cells send to PD-1 via the PD-L1 molecule to “trick” the T-cell into recognizing the cancer cell as normal. This then allows the immune system to attack and kill tumor cells.
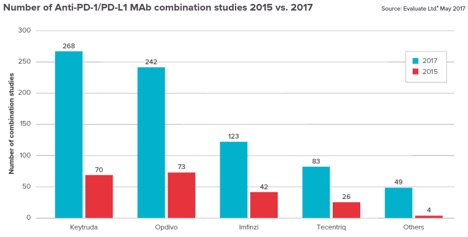
Biotech and pharma pipelines are filling up with combination therapies for anti-PD-1 and PD-L1 antibodies. According to the Cancer Research Institute, there are now over 1,500 clinical trials testing the popular checkpoint inhibitors (up from 215 trials in 2016).
Checkpoint inhibitors bring new hope to patients that previously had no alternative after chemo failed. Nivolumab, marketed as Opdivo, is a programmed death receptor-1 (PD-1) blocking antibody that began receiving FDA approvals in 2014. It’s now used for the treatment of advanced melanoma, advanced non-small cell lung cancer, advanced small cell lung cancer, advanced renal cell carcinoma, classical Hodgkin lymphoma, advanced squamous cell carcinoma of the head and neck, urothelial carcinoma, MSI-H or dMMR metastatic colorectal cancer, and hepatocellular carcinoma. Pembrolizumab, marketed as Keytruda, has shown dramatically higher survival rates in lung cancer patients.
Even with the success to-date, there is a lot of work required to improve the quality of these treatments. Checkpoint inhibitor treatments can run upwards of $150,000 (and other up-and-coming cancer immunotherapy treatments such as CAR-T cell therapy, can carry price tags up to $400,000).
Most checkpoint inhibitors are effective in only in a small percentage of patients. The reasons why are still unknown, though research is indicating that they tend to be effective in patients whose cancer cells have a higher number of mutations.
Biotech and pharma are searching for broader solutions by combining checkpoint inhibitors with other cancer treatments, hoping that their own therapies will improve the safety and efficacy of the successful PD-1 and PD-L1 inhibitors already on the market. But this explosion of research in the clinic exposes more researchers and patients to the unexpected side effects that are showing up in clinics around the world.
One of the main challenges with immunotherapy treatments occurs when the immune system starts to attack the patient’s own body. A 2016 study revealed that 30% of patients in a PD-1 therapy study experienced “unexpected side effects,” with a quarter of the reactions described as severe, life-threatening or requiring hospitalization. When the new drugs were used in combination, more than 50% of the patients struggled with severe side-effects.
Patients undergoing immunotherapy treatments may experience reactions like dermatitis (from rashes to toxic epidermal necrolysis), pancreatitis, immunitis, iridocyclitis, hepatotoxicity, hypophysitis, neuropathies, nephritis and hepatitis. Some patients with underlying autoimmune disorders can experience exacerbations of underlying autoimmune conditions when on therapies blocking CTLA-4 and PD-1. These reactions often start out mild but can become severe and life-threatening if not recognized.
Therapies targeting checkpoint receptors have shown to increase toxicities that can create a unique set of adverse events called immune-related adverse events (irAEs). Since multiple studies have shown that there is an association between irAEs and clinical benefit, it’s important to have a comprehensive approach for evaluating and treating SAEs.
For every oncology immunotherapy study we recommend the following:
Training for Early Recognition
Early recognition and AE management is critical to allow the patient to continue on the study treatment per the protocol. This can be addressed during site training by educating the study coordinator research nurses about potential immune-related AE symptoms.
Combination Therapy Evaluation
Each therapy’s relationship with the AE must be assessed. Studies have shown that algorithms designed for specific therapies can help clinicians manage and treat the most common irAEs associated with the treatment. These can often predict the irAE frequency and severity based on dosage and frequency of the treatment.
Specialized Report Forms
Adverse event report forms must be designed in a way that addresses the possibility for multiple attributions. For example, if there is more than one drug being given, the case report form (CRF) needs to ask about the AE’s relationship to each individual drug, rather than the study treatment as a whole.
CRF Guidelines
Case report guidelines must include specific instructions for the site on how to record the AE.
Medelis has been managing checkpoint inhibitors and CAR T-cell therapy studies since 2008 – so we’ve been able to work with many sites in the U.S. and Europe to develop the safety SOPs and train them.
Connect with us to learn more, download our guide Choosing the Right Immuno-oncology CRO or listen to our webinar: How Immune-Response Criteria Is Changing Immunotherapy Treatments.