With the ASH 2018 annual meeting right around the corner (in sunny San Diego, a great place to be in December), it is a good time to highlight the progress of immunotherapy treatments in hematology.
Acute myeloid leukemia (AML) is one disease that has essentially relied upon the same treatment for the past 40 years. This current standard, intensive induction therapy followed by hematopoietic stem cell transplantation, is somewhat effective in patients under 60 years of age, but far less effective for elderly patients. Thus, many researchers have been focusing on small molecules and immunotherapies to find more efficacious treatments for AML.
One class of immunotherapeutic agents that is showing promise for AML are bispecific antibodies. Bispecific antibodies (BsAbs) are capable of binding two targets simultaneously and can potentially induce a powerful anti-tumor immune response.
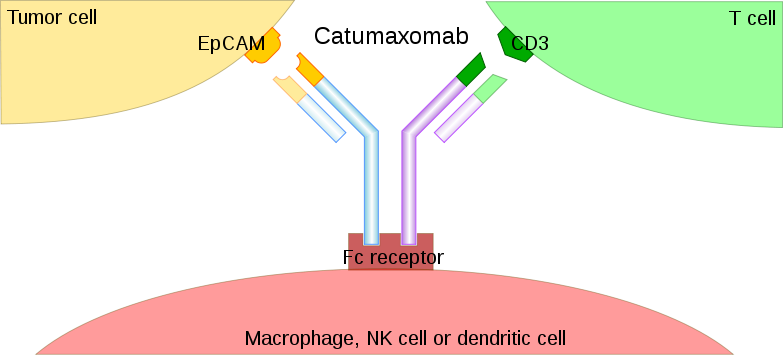
Bispecific antibodies were described in the 1960s, with the first monoclonal BsAbs generated in the 1980s. The first article describing the therapeutic use of BsAbs was published in 1992, with trifunctional hybrid antibodies (Triomab) being introduced in 1995. The Triomab platform offered a solution to the random association of heavy/light chains observed in classic quadroma technology by combining the halves of two distinct antibodies into a full-size, functional mAb. Research then slowed and didn’t pick up until 14 years later, when two Triomabs were approved for therapeutic use: catumaxomab in 2009 and blinatumomab in 2014. Recent papers have focused on generating BsAb via genetic engineering and chemical conjugation and their performance in preclinical and clinical studies.
The dual specificities of bispecific antibodies – one for a tumor-associated surface antigen and the other for a surface antigen on the effector cells (T cells or NK cells) – bring tumor cells into close proximity to the effectors. This is positive for AML because, if the binding to the second specificity is agonistic, the cytotoxic functions of effectors can be activated at close proximity to the leukemic cells.
Although some targeted therapies for heme (such as FLT3 inhibitors) have demonstrated encouraging results in early clinical trials, their benefit is restricted to a small portion of patients. Blinatumomab, a bispecific T-cell engager (BiTE) targeting human CD3 and CD19 for relapsed/refractory acute lymphoid leukemia (ALL) was approved by the FDA in 2014.
More recent studies using bispecific antibodies that redirect the cytotoxic activity of effector T cells by binding to CD3, the signaling component of the T-cell receptor, and a tumor-associated antigen, could provide new treatment options for AML.
Researchers are using various combinations of whole antibodies and their fragments to yield more than 60 different formats of AML bispecific antibodies. These include:
There are advantages and disadvantages with all of these AML-associated antigens for antibody development. Moving forward, the focus on immunotherapy is the key, not focusing solely on a specific combination such as CAR T-cell therapy + PD1.
Since we’re in the early stages of studying these types of therapies in the clinic, many sites, CROs and clinical operations groups are still defining the best approaches for keeping patients safe. As immunotherapy treatments become more prevalent, clinical teams will become more accustomed to handling the side effects.
As a specialty oncology CRO, Medelis has been managing immunotherapy studies including checkpoint inhibitors and CAR T-cell therapy studies since 2008. We’ve been able to work with many sites in the U.S. and Europe to develop the safety SOPs and train them and wish to share our knowledge to bring more of these therapies to market.
Connect with us to ask a question or discuss your study.